flowchart TD A[1. Import data, event markers, channel locations into EEGLAB] --> B[2. Down-sample EEG data to 256 Hz] B --> C[3. Apply Finite Impulse Response filter: 1-40 Hz ] C --> D[4. Remove non-relevant channels - EOG, Mastoids] D --> E[5. Manually remove channels with uncommonly high or low power] E --> F[6. Re-reference data to common average] F --> G[7. Interpolate removed electrodes, using spherical interpolation]
Sleep restriction affects vigilant attention:
Behavioural and neural correlates
University of New England, NSW
Talk information
- This talk was written in Quarto (R Markdown/R Studio) & is available online
Stages of sleep
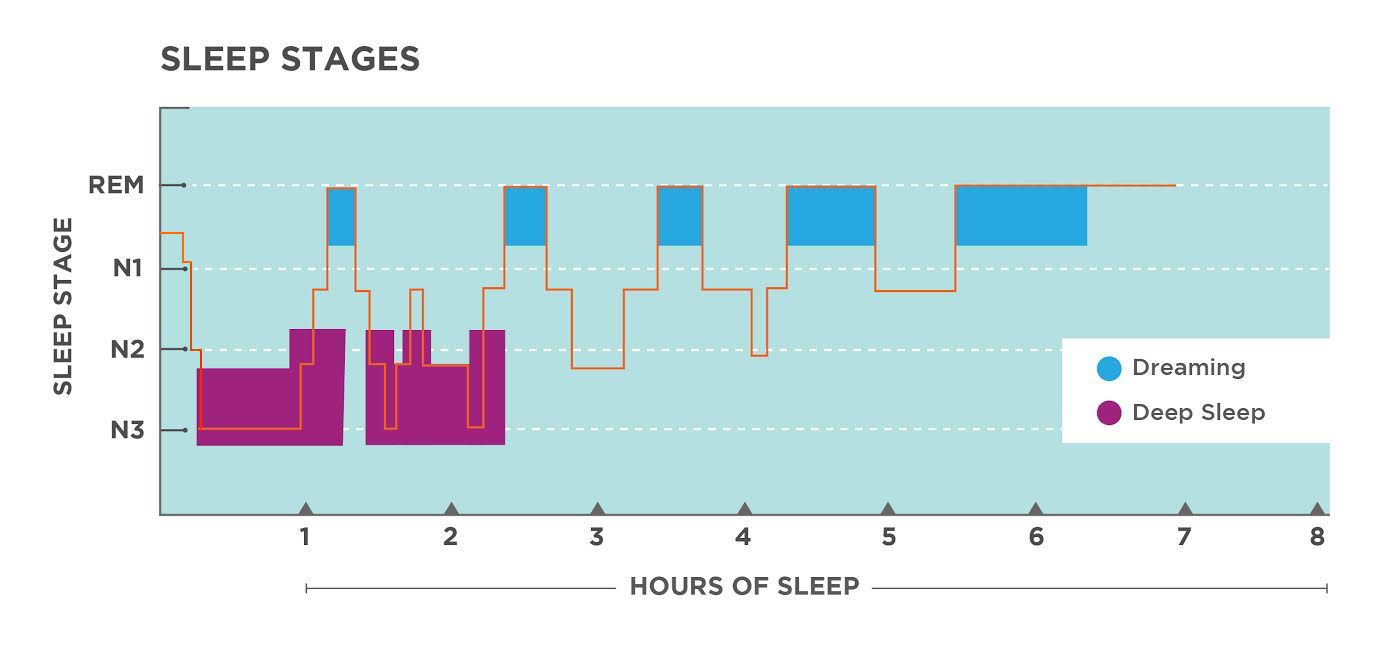
- Staging sleep involves classifying the EEG signal, often still by hand
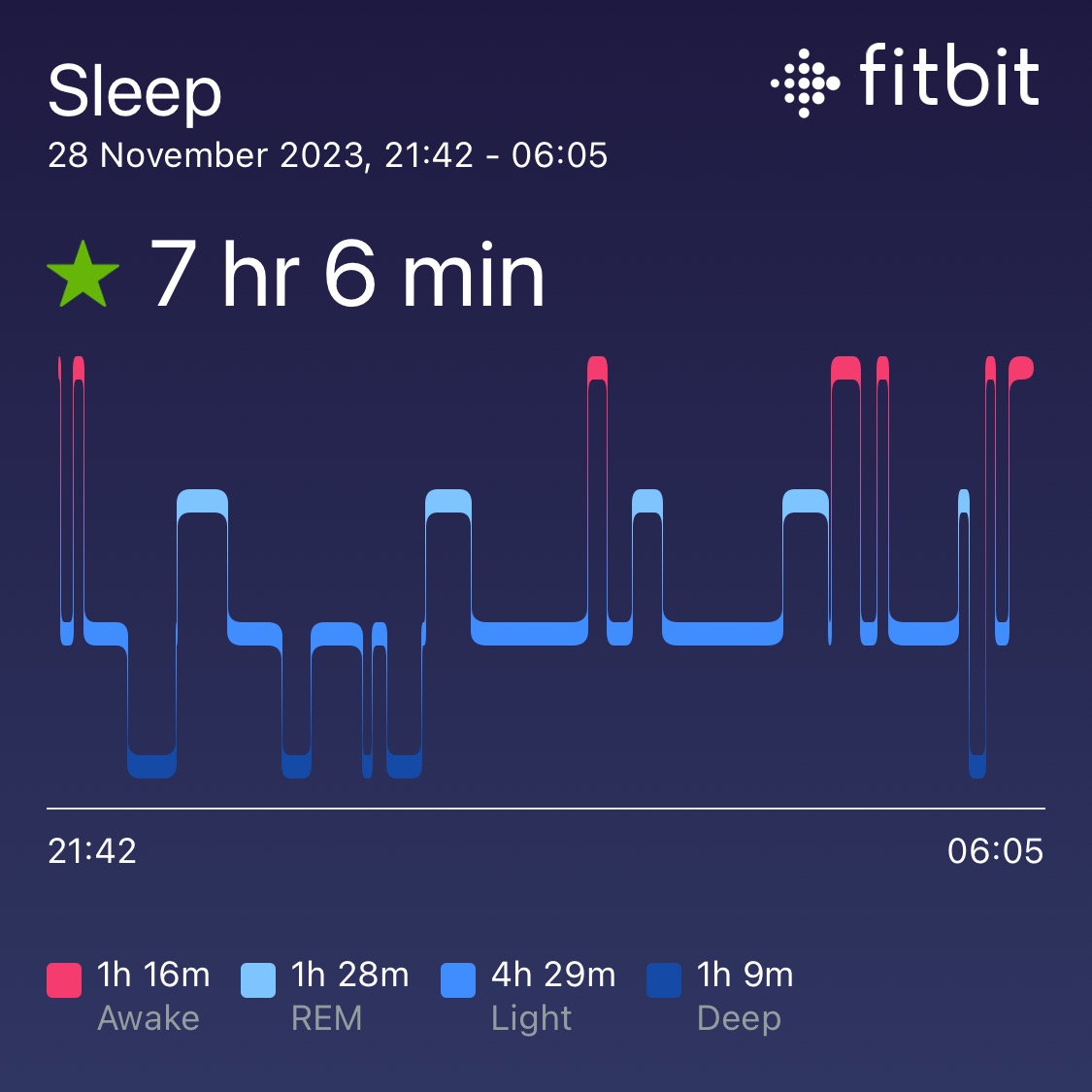
- Modern wearable technology enables much easier sleep tracking
- Accuracy not as good as polysomnography, but improving
- Enables inexpensive in-home sleep assessments
- Big data?
- Also relatively cheap
Sleep restriction vs. sleep deprivation

- Much research looks at total sleep deprivation (no sleep overnight)
- Profound effects on cognition (memory, attention, etc)
- But sleep restriction (less sleep than usual) is much more common
- Effects on cognition not as well researched
- But this type of sleep loss is much more common!
Background
- These data were originally collected in 2015 as part of an Honours project by Lucienne Shenfield at ANU
- Now working as a clinical psychologist specialising in sleep
- Some of this work is now published
- What we’ll mainly talk about is the work of another Honours student Lourdes Machin (UNE, 2022)
- Lourdes analysed the during-task EEG data for the Psychomotor Vigilance Task


Shenfield, L., Beanland, V., Filtness, A., & Apthorp, D. (2020).
The impact of sleep loss on sustained and transient attention: An EEG study. PeerJ, 8, e8960.
Background
- CNS centres regulating sleep overlap with those regulating attention & arousal
- Disrupting sleep disrupts attention
- But what is attention?
- Catch-all term for multiple processes
- Here we focus on sustained attention/vigilance
“Attention is psychology’s weapon of mass explanation” - David Burr, personal communication
Participants
Inclusion criteria: 18 – 65 years; regular sleep 7-8 h/night
Exclusion criteria:
- Regular smokers
- Shift work
- High caffeine consumption (> 5 cups/day)
- Diagnosed ADD or sleep problems
- Recent head injury or history of seizures
Final sample: 25 participants (15 females)
- Age: 21 – 55 (M – 24.79 years, SD = 6.82)
Experimental design
- Within-subjects: Normal sleep (NS) and Sleep-restricted (SR)
- Sleep restriction: Delay bedtime by 3 hrs, set alarm for 5 hrs later
- Counterbalanced order
- Sleep monitoring: Sleep diary and FitBits
- Tasks: Attentional Blink (AB) and Psychomotor Vigilance Task (PVT)
- EEG: Resting state and ERP (during tasks)
Sleep restriction
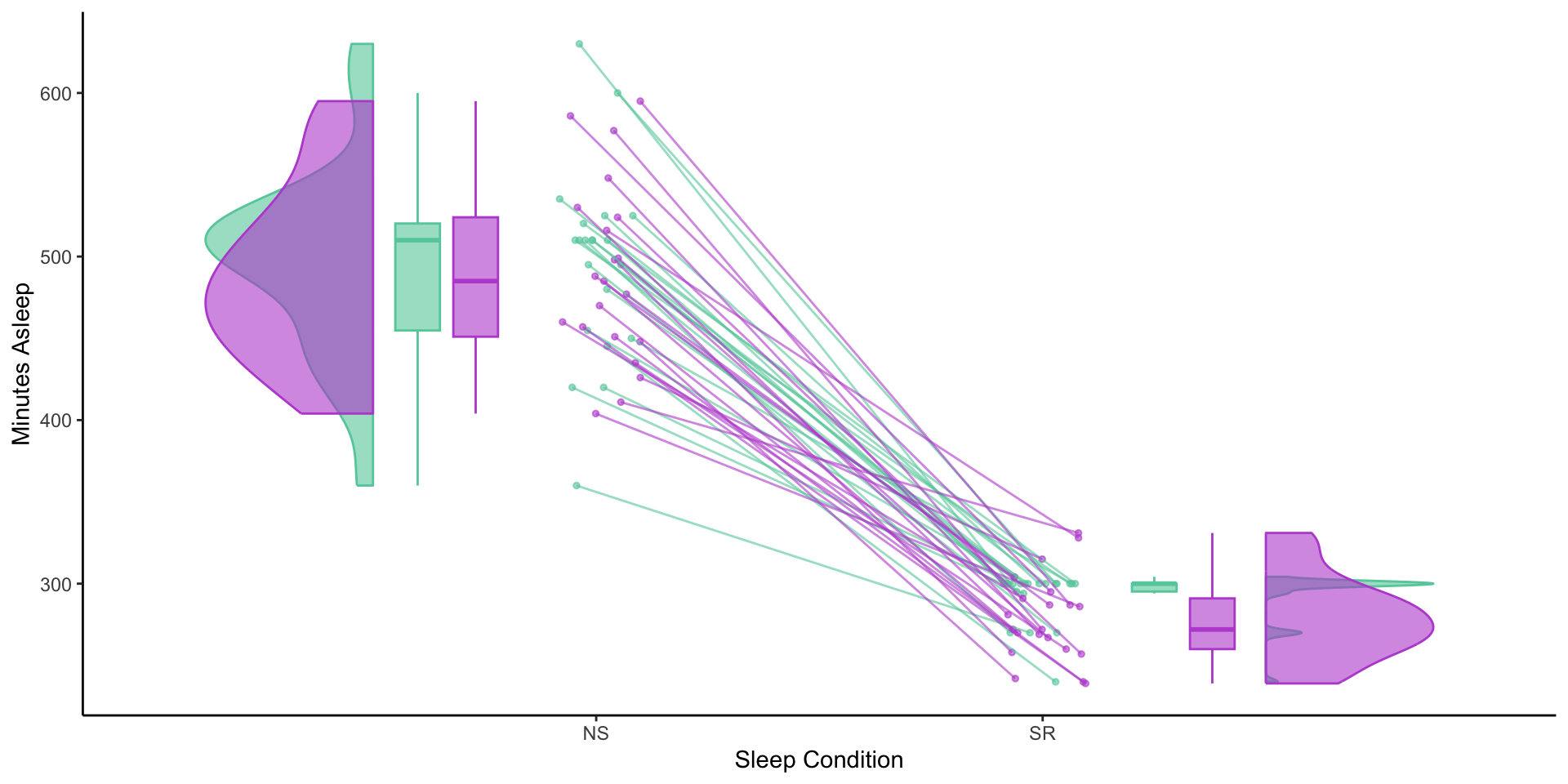
Minutes of Sleep: Diary and FitBit
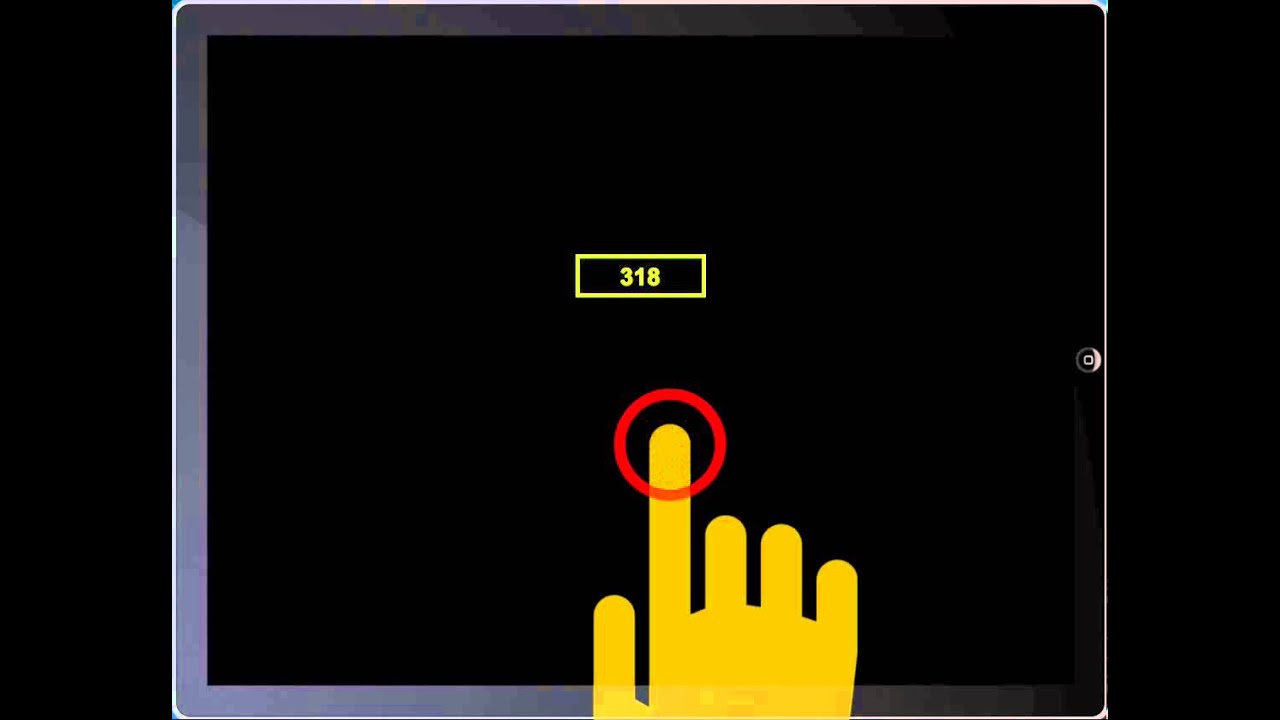
Task: Psychomotor Vigilance Task
- 10 minutes long
- Participants press a button as soon as they see red numbers appear
- Numbers appear at random intervals (2-10s apart)
- Reaction time (ms) is measured
- Also lapses (>500ms), false starts (<100ms)
- ~80 trials per session
EEG
- Compumedics NuAmps 32-channel EEG system
- Electrodes 10/20 system
- Electrode maximum impedance: 5 \(k\Omega\)
- Signal recorded at 1000 Hz
- NuAmps digital amplifier
- Curry 7.0.9 software
- Resting state and during-task recording

EEG preprocessing
flowchart TD A(8. Visual inspection of channel plots. Removal of large artifacts ) --> B(9. Run independent components analysis ) B --> C(10. Run ICLabel - Remove muscle and eye IC artifacts ) C --> D(11. Remove remaining blink-like ICs with probability between 0.8 and 0.9 ) D --> E(12. Extract epochs of 3s length for ERP and time frequency analysis ) E --> F[13. Locked to stimulus onset: -1s, +2s ] E --> G[14. Locked to stimulus onset: -1.5s, +1.5s ]
Behavioural data
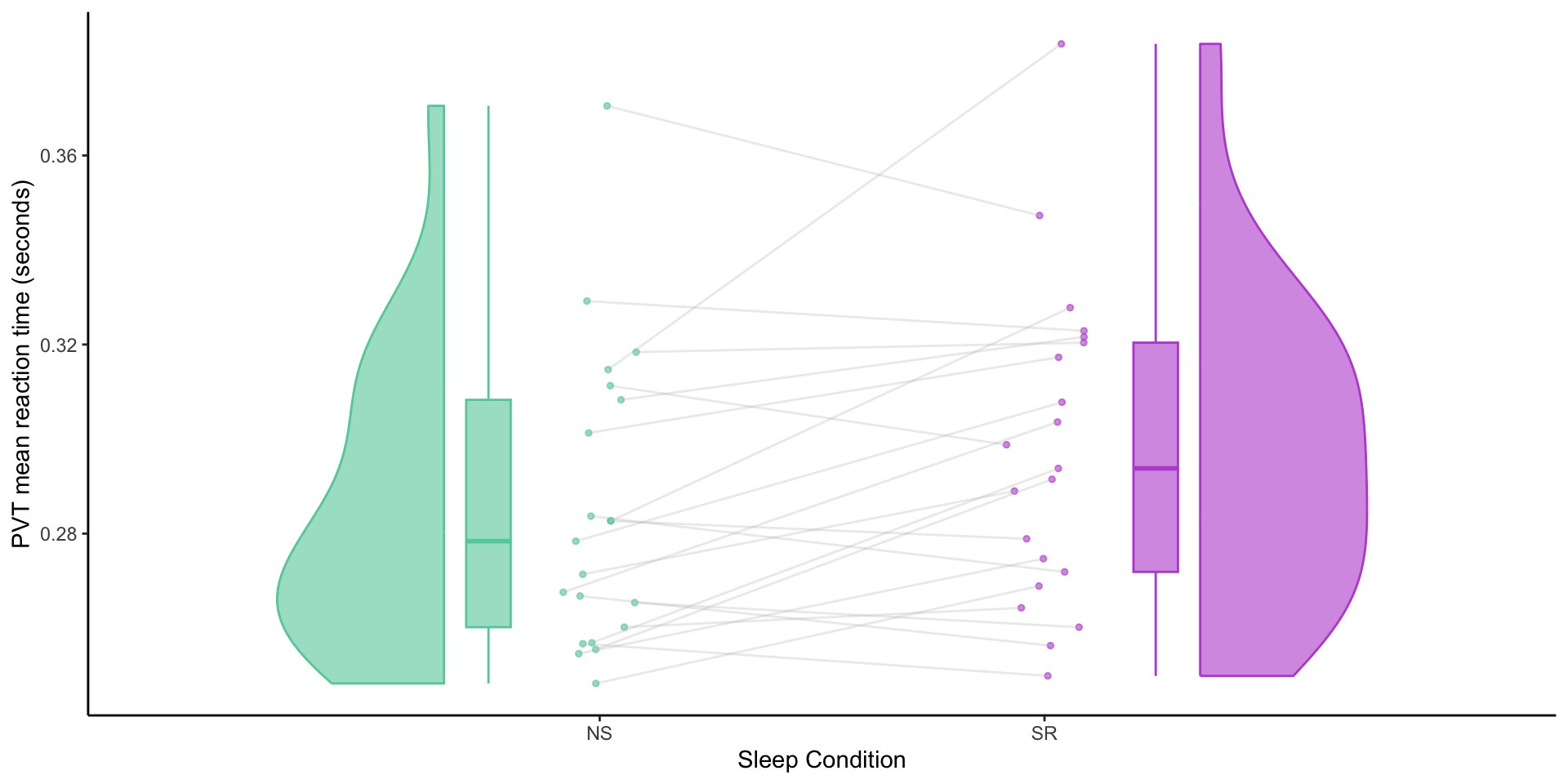
Mean reaction times by sleep condition
Subjective sleepiness
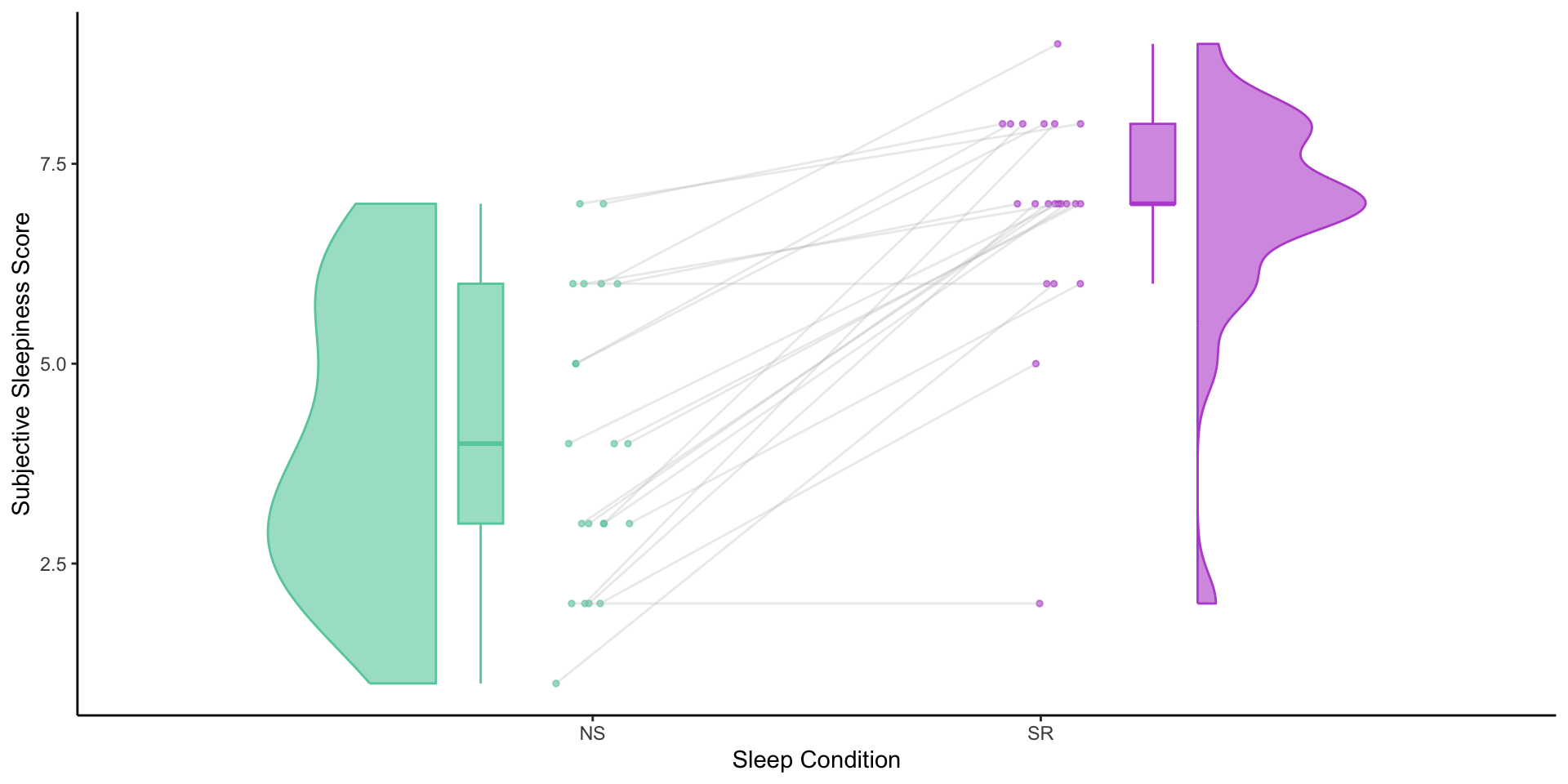
Mean Karolinska Sleepiness Scale scores by sleep condition
ERP data
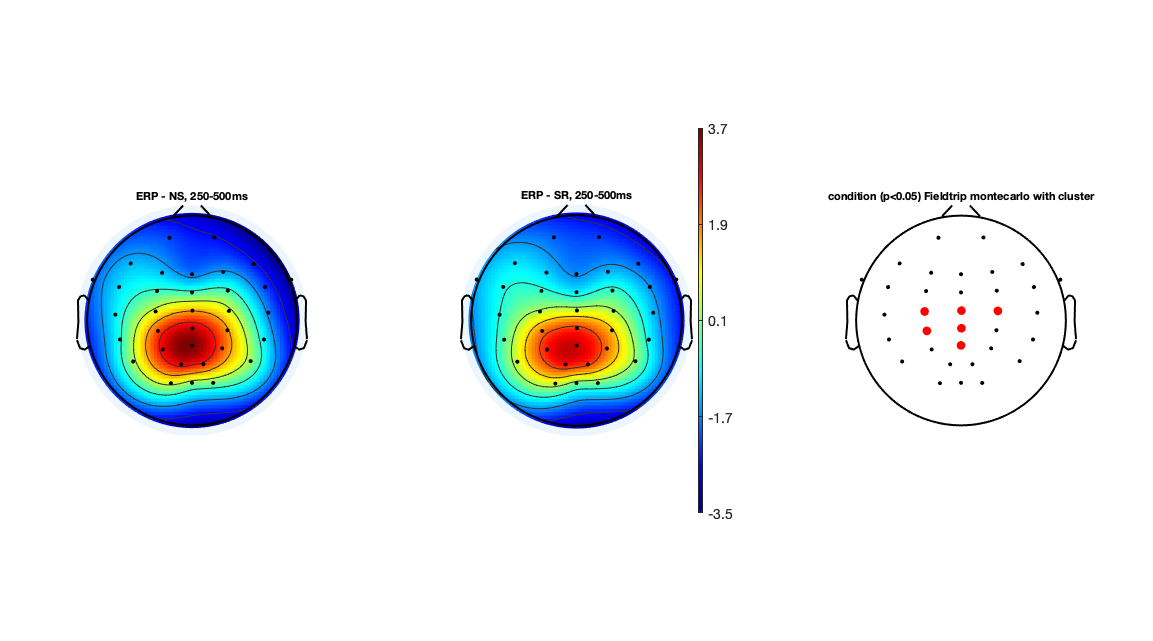
ERP amplitude differences: ERP Topographic Maps 250-500 ms
ERP data - differences - Pz
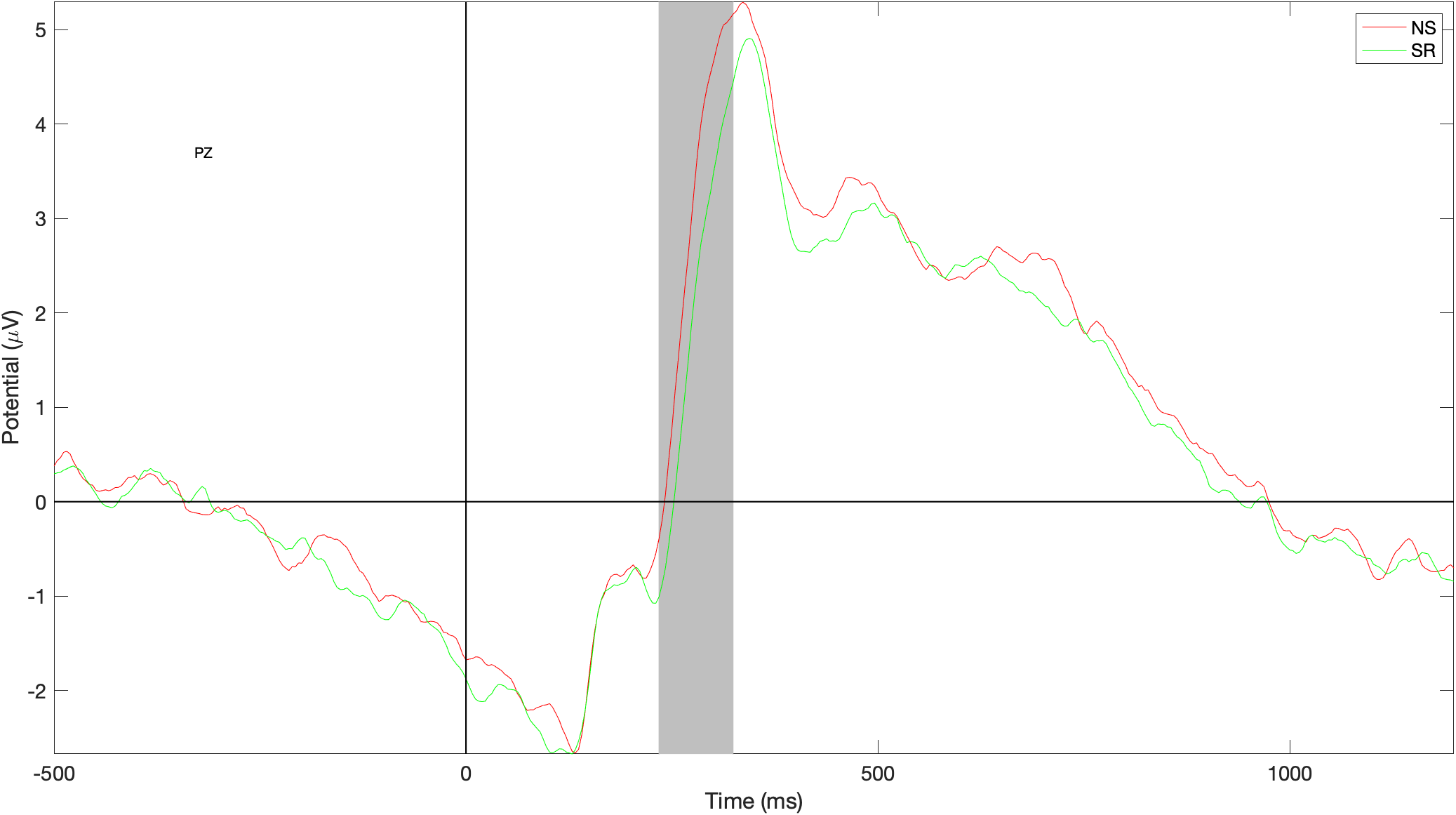
Grey bar shows statistically significant differences in amplitude at the start of P3
ERP data - differences - CPz
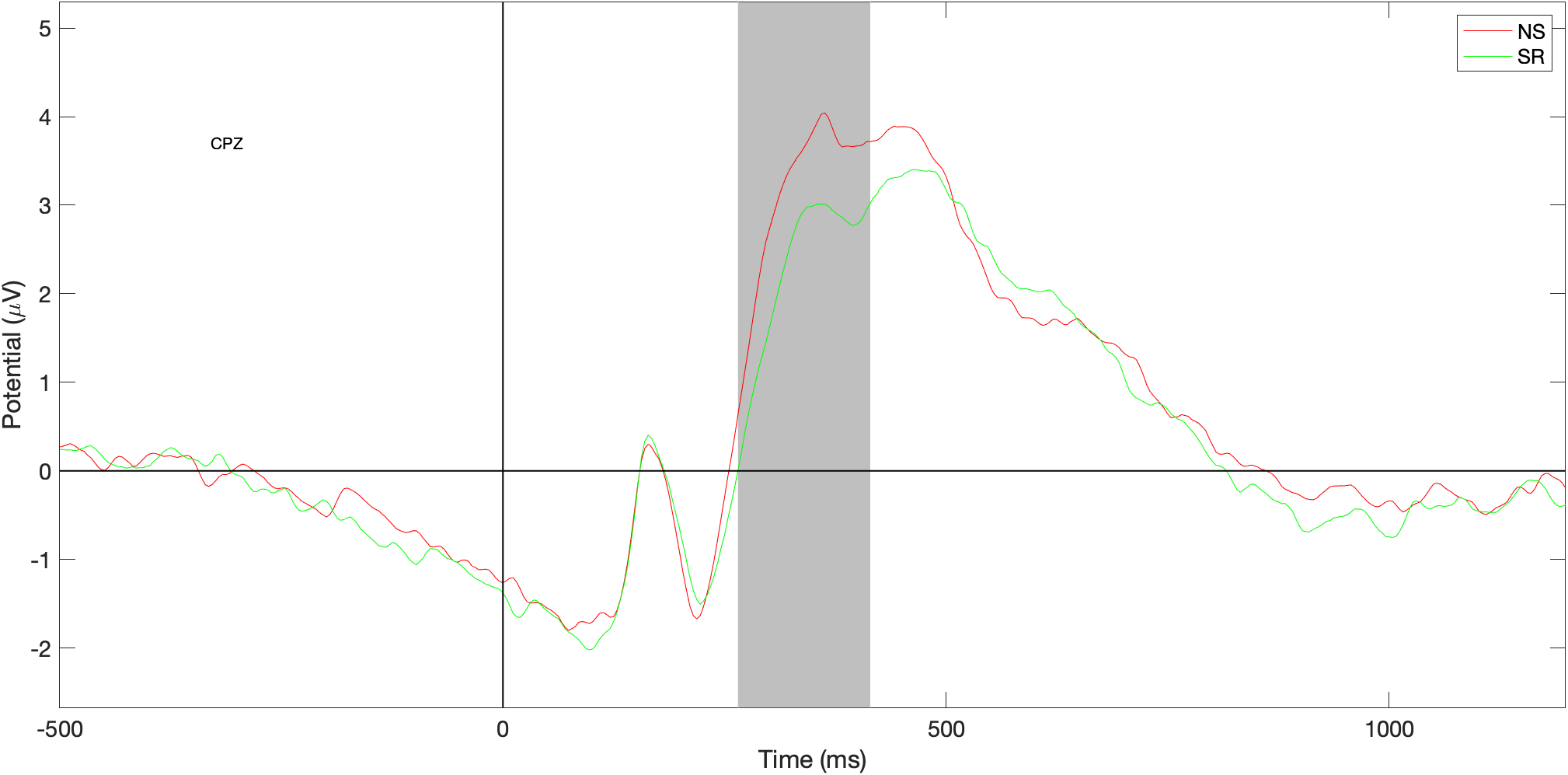
Grey bar shows statistically significant differences in amplitude at the start of P3
Source localisation - NS - SR

eLORETA Source Localisation of Current Source Density Differences (from ERP) between NS and SR
Source localisation - NS - SR
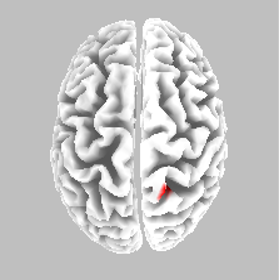
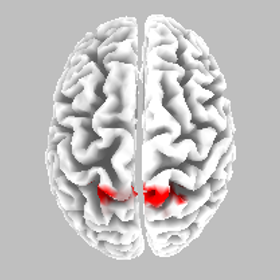
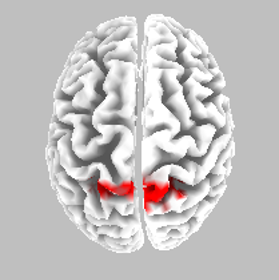
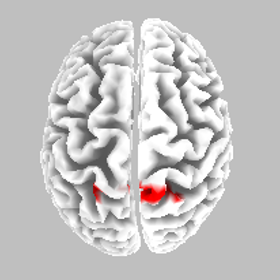
Best Match - Brodmann area 5, postcentral gyrus, parietal lobe
Somatosensory cortex - motor preparation affected?
Resting state data
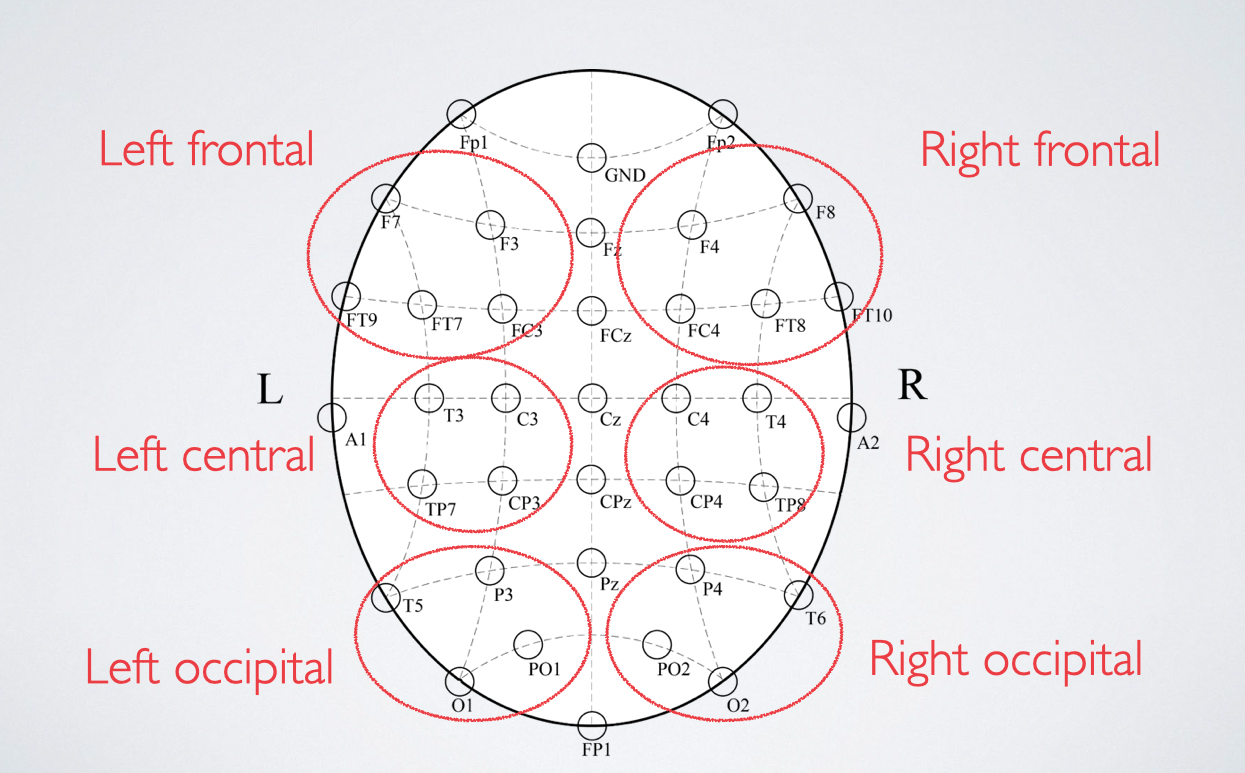
- We also measured resting state EEG (eyes open and closed)
- Divided into regions of interest (ROIs)
- L & R frontal, central, occipital
- Significant increases in alpha & decreases in delta relative frequency after sleep restriction
- Most prominent in right central ROI (C4, T4, CP4, TP8)
Resting state changes - right central ROI, eyes closed
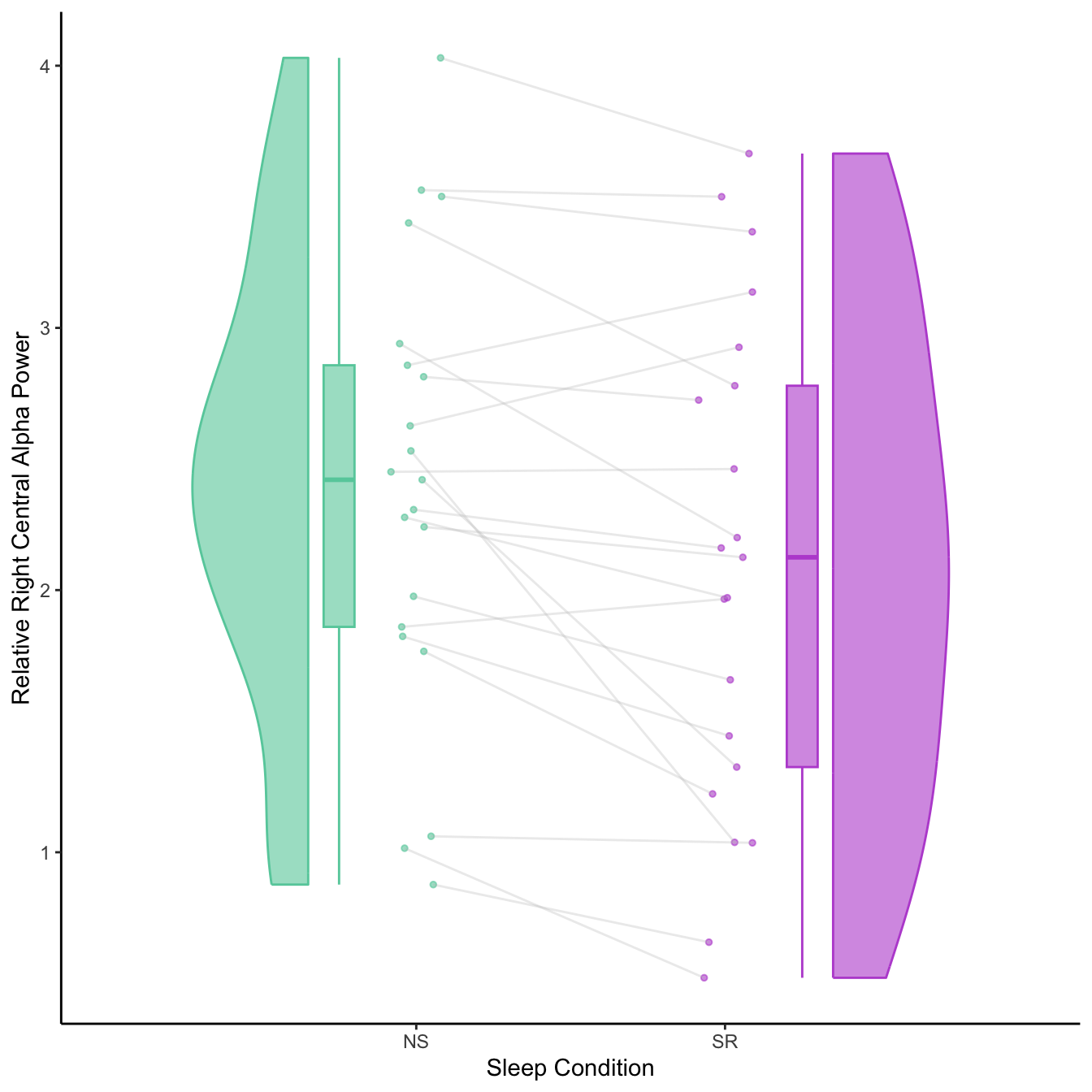
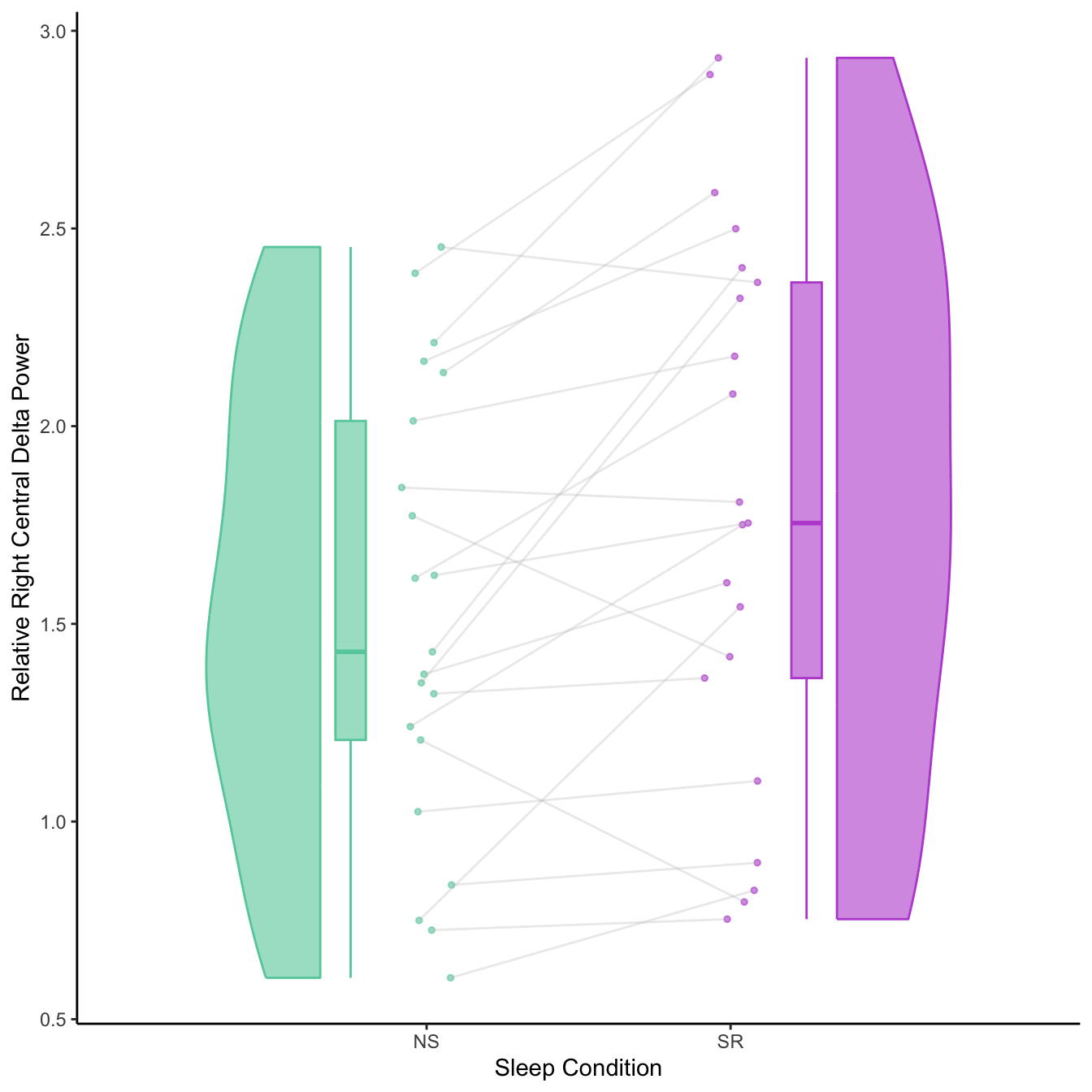
Individual variation in effects of sleep restriction
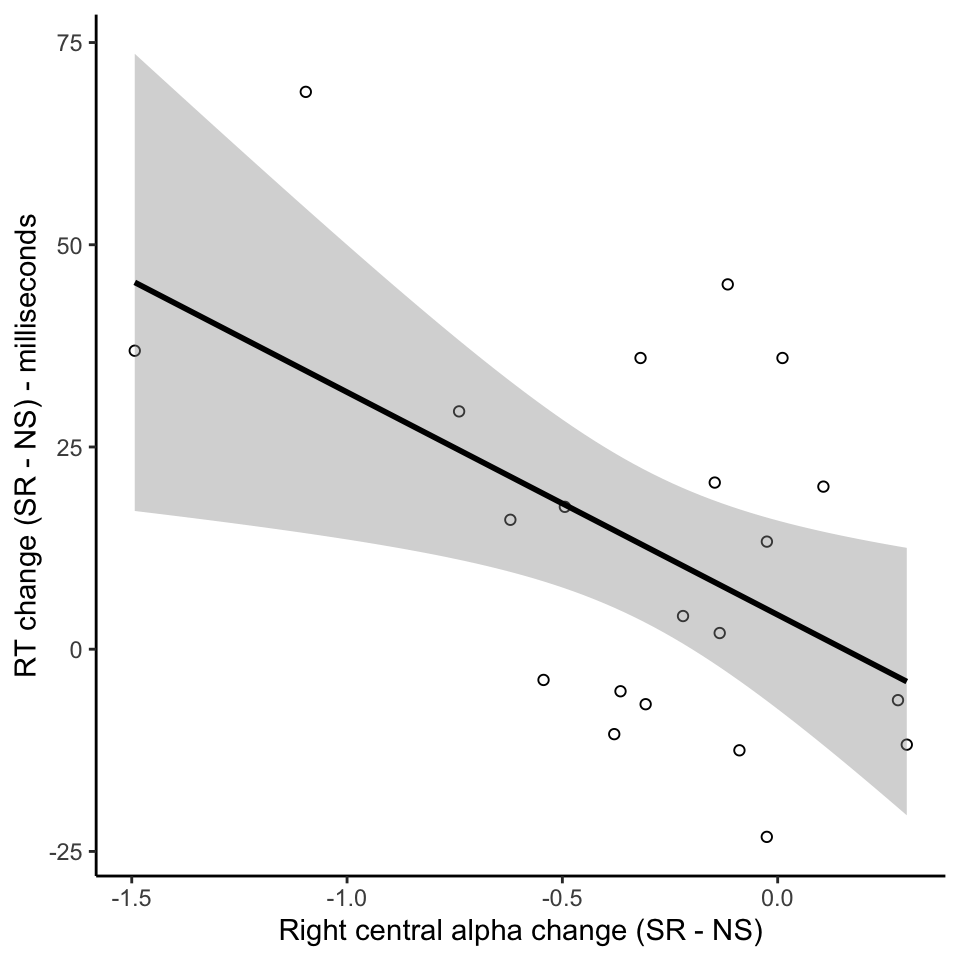
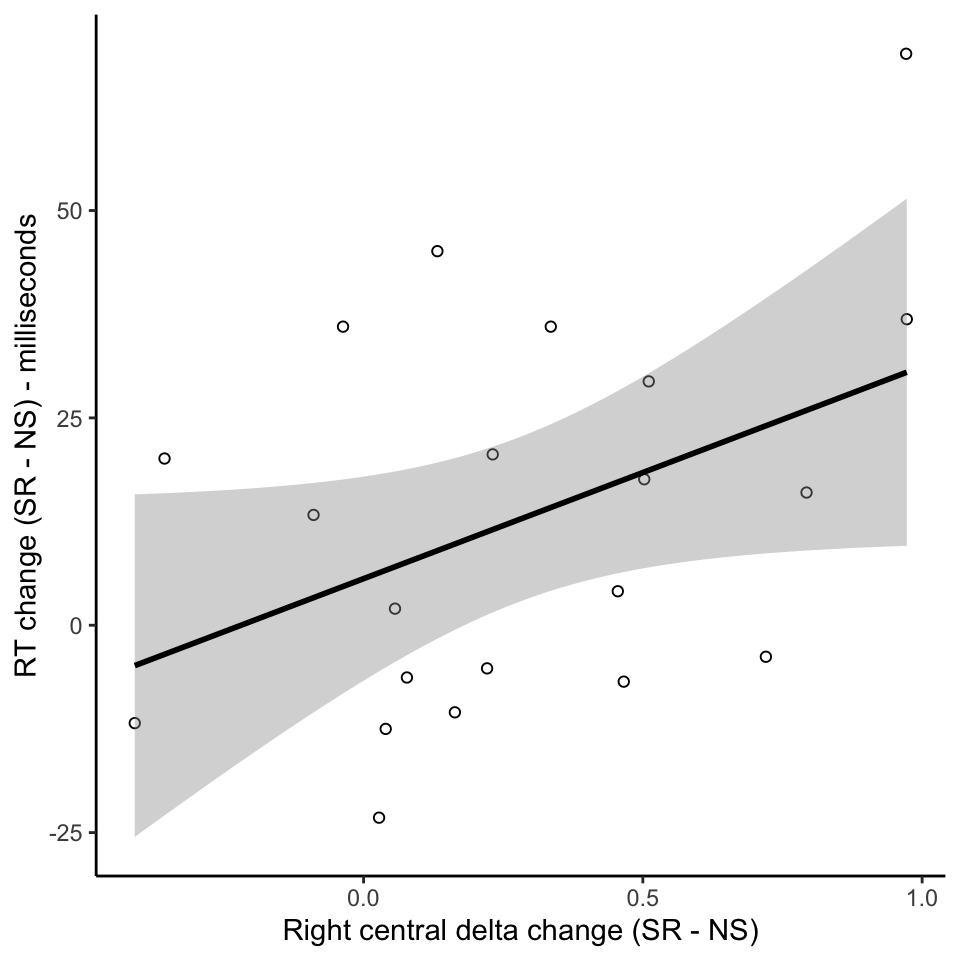
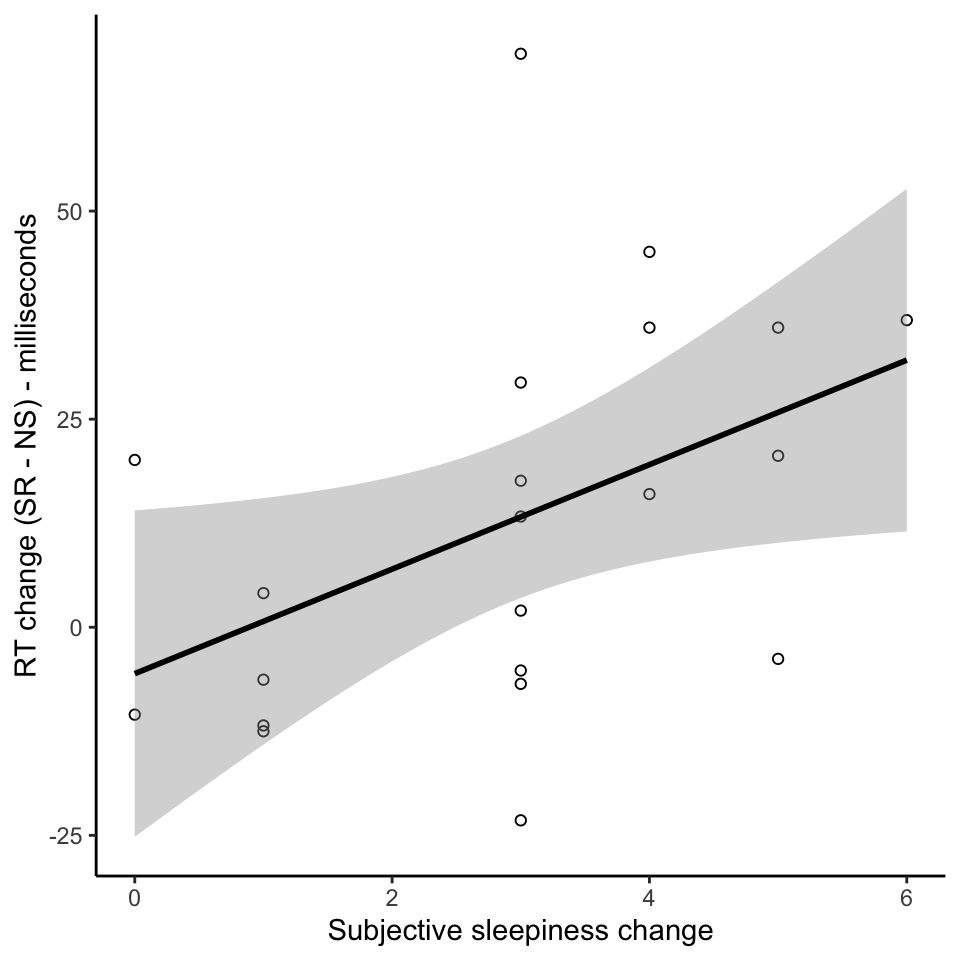
Conclusions
- Mild sleep restriction caused changes in resting state frequencies in right central areas
- Small increase in reaction times in a vigilance task (PVT)
- Increase in ERPs (P3) at central sites during the PVT
- Source localisation using eLORETA suggests the post-central gyrus (Brodmann Area 5) as the origin of this difference
- This area has not previously been associated with sustained attention - motor preparation/execution differences?
- Individual variations in effects of sleep restriction - behavioural and neural effects correlate
Questions?

